Adorable! Why Does Steam Have A Lower Specific Heat Than Water
If one pound of water drops one degree Fahrenheit in temperature it produces approximately 1 British thermal unit BTU of heat. Since it is a kettle on your stove the pressure is atmospheric.
Steam also does not change state while it is used as a heating medium and it gives up heat energy to the secondary medium.
Why does steam have a lower specific heat than water. Nuclear Engineering 2 points 4 years ago. Because the specfic volume of water is several orders of magnitude lower than that of steam the droplets of water in wet steam will occupy negligible space. Up to 20 cash back Ryan.
A similar amount of heat energy would increase the temperature of dry land to a much higher temperature and the soil or dirt would keep the heat from going into the ground. This is because the specific enthalpy of evaporation decreases as the steam pressure increases. This phenomenon can be explained by Daltons Law of Partial Pressures.
If we use an example though youd find that. As it gives up heat energy to the secondary medium its temperature drops. The real standout in molar heat capacities are substances like liquid water.
Air can diffuse and mix with steam at start-up or during regular operation which effectively reduces the steam pressure and results in reduced heat transfer. I dont understand why. To me it seems that the bonds between water molecules in a solid are stronger and hence require a greater deal of thermal energy to break.
If we select a steam-to-water heat exchangeralso called a convertorat 30 PSIG entering steam pressure we get a shorter unit than if we select it at 5 PSIG entering steam. Specific heat of water. If you compare molar heat capacities then Al is about the same as any other metal eg.
Theres slightly more latent heat in higher pressure steam but the overriding factor comes from the steam temperature. It isnt hotter. Hence the PE of the system increases.
Superheated steam has a lower density so lowering the temperature does not revert it back to its original liquid state. Because the specific volume of water is several orders of magnitude lower than that of steam the droplets of water in wet steam will occupy negligible space. This means fewer ways of distributing the energy as the temperature increases that is lower dSdT meaning lower heat capacity.
This is called superheated steam. The number of hydrogen bonds is not the reason for waters high heat capacity but because of their bending modes of vibration that dont exist in ice because its too rigid nor in steam as theres no intermolecular bonds. So superheated steam is not as effective as saturated steam for heat transfer applications.
0444 JGram Degree Celsius iron has a lower specific heat than water. 30 PSIG steam is 274F while the 5 PSIG steam. The point at which the saturated water changes to saturated vapor is dependent on the pressure.
Wet steam will have lower usable heat energy than dry saturated steam. This is one of the highest. Anything with a low density will have fewer degrees of freedom per gram by definition and therefore a lower specific heat.
It is especially effective to transport heat through vaporization and condensation of water because of its very large latent heat of vaporization. In a steam radiator steam at 110C condenses and the liquid water formed is cooled to 80C. More heat energy is necessary to heat up the area because the water absorbs the energy.
It seems to me that it ought to be a poor choice because. Deserts reach extremely high temperatures specifically because of their lack of water. Saturated steam still has boiling that goes on and still has condensate to boil.
The specific heat of water at 25 degrees Celsius is 4186 joulesgram degree Kelvin. For example the specific heat of iron is 449 JkgC sand is 830 JkgC and oak timber is 2400 JkgC. Thats because water comprised of two hydrogen atoms and one oxygen atom is electronegative.
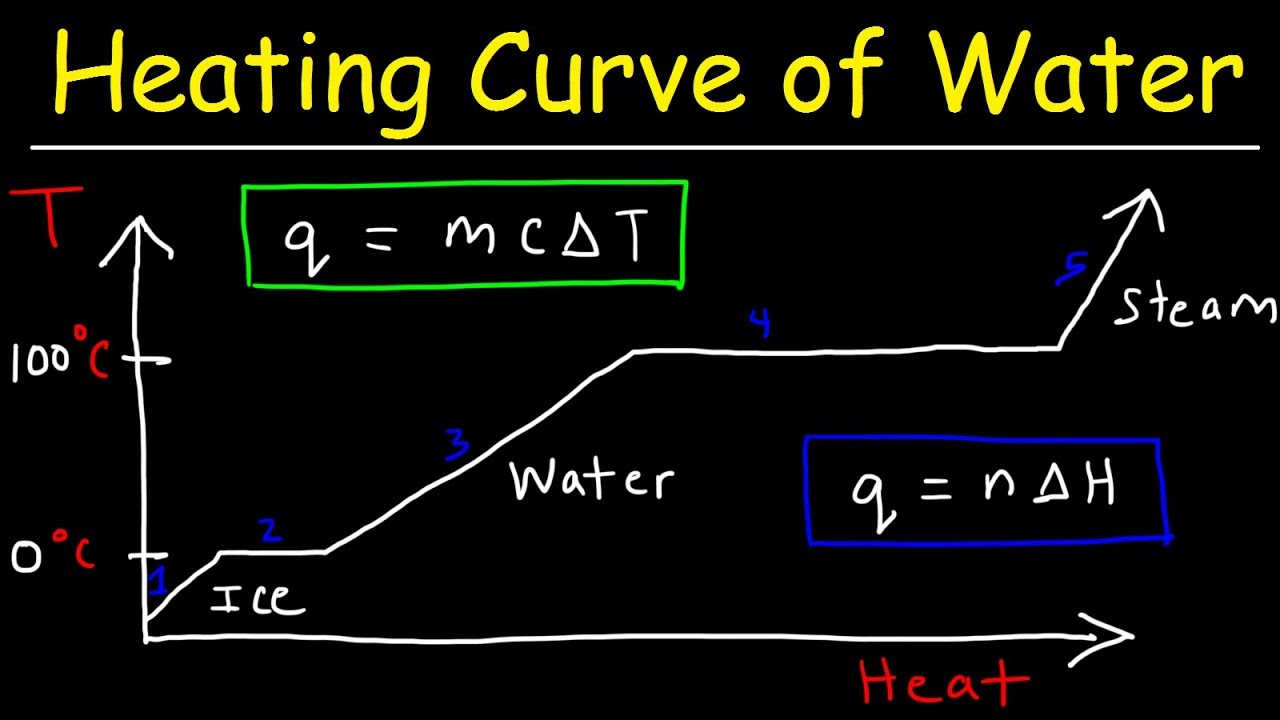
The water transitions to saturated vapor steam at 100 C so unless the. It requires less heat per unit mass to create a greater change in temperature for metal than it does for water. Water has a high specific heat.
Specific heat capacity is heat capacity per gram. Creates temperature gradients over the heat transfer surface as it cools to saturation temperature. The steam phase diagram.
Liquid has better heat transfer properties than liquid and boiling has amazing heat transfer properties for lower temperatures. Therefore the specific volume of wet steam will be less than dry steam. The specific heat capacity of water at -10 degrees Celsius ice is 205 joulesgram degree Kelvin.
Water and steam are a common fluid used for heat exchange in the primary circuit from surface of fuel rods to the coolant flow and in the secondary circuit. The specific heat capacity of water at 100 degrees Celsius steam is 2080 joulesgram degree Kelvin. Provides lower rates of heat transfer whilst the steam is superheated.
This is general of course since there are different types of metal. Al 243 JK-mol Cu 245 JK-mol and not very different from air eg. Requires larger heat transfer areas.
Actual specific volume V g X Where V g is the specific volume of dry saturated steam. Steam gas-phase H 2 O is very commonly used as the working fluid for external-combustion heat engines. The heat energy enthalpy of evaporation needed by the water at higher pressure to change it into steam is actually less than the heat energy required at atmospheric pressure.
Dropping the temperature of saturated steam however will revert it back to its old form of water droplets. Latent Heat of VaporizationWhy does steam cause more severe burns than boiling waterIt is because steam is jealous of boiling waterNoIt is because. Therefore the specific volume of wet steam will be less than dry steam.
Unless I am seriously confused about something you have no choice but to throw the working fluids heat of vaporization away every time around a Rankine cycle. It used due to its availability and high heat capacity both for cooling and heating. That means for every 4184 J of energy added to one gram of water the temperature of the water will increase by one degree Celsius.
4186JGram Degree Celsius Specific heat of say iron. The specific heat of water is quite a bit higher than many other common substances. Throughout my time doing physics I have noticed that ice has a lower specific heat capacity than water.
Because of the high specific heat of water water and land near bodies of water are heated more slowly than land without water. In a steam radiator steam at 110 C condenses and the liquid. Up until you hit critical heat flux and go through transition boiling.

Heating Curve And Cooling Curve Of Water Enthalpy Of Fusion Vaporization Youtube
Why Is The Specific Heat Capacity Of Water Exactly Twice That Of Ice Quora

Specific Heat Of Water Video Khan Academy

Specific Heat Capacity Of Materials The Engineering Mindset
Why Does Water Have Such A High Specific Heat Capacity Quora
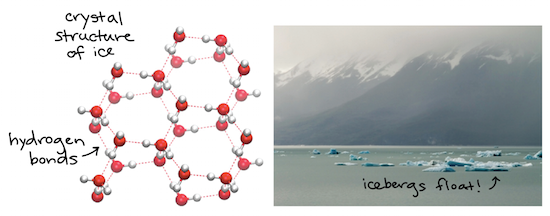
Specific Heat Heat Of Vaporization And Density Of Water Article Khan Academy
Why Is The Specific Heat Of Water Greater Than The Oil Quora
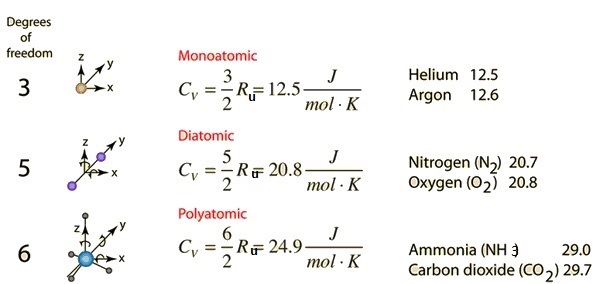
Properties Of Common Gases Steam And Moist Air With Temperature Mhi Inc

Heat Energy And Transfer Latent Heats Of Fusion And Vaporisation Hvac Machinery

Icse Solutions For Class 10 Physics Specific Heat Capacity And Latent Heat A Plus Topper
What Are Some Real Life Examples Of Specific Heat With Explanations Quora

What Is The Specific Heat Of Water How Is It Special

Heat Energy And Transfer Specific Heat Capacity Hvac Machinery

Variations Of The Isobaric Specific Heat Capacity C P Of Water Vapor Download Table
Specific Heat And Phase Change Read Physics Ck 12 Foundation

Can Anyone Suggest A Material With The Highest Specific Heat Capacity Higher Than Water




