Helpful! Why Are Covalent Bonds Important To Life
Carbohydrates illustrate the importance of subtle differences in covalent bonds in generating molecules with different biological activities. Which of the following properties of water molecules has important implications to life.
Single And Multiple Covalent Bonds Article Khan Academy
A dipole is a molecule that is electrically neutral.

Why are covalent bonds important to life. DNA has a double-helix structure because hydrogen bonds hold together the base pairs in the middle. Hydrogen bonding is important because it is crucial to all life on Earth. However several types of noncovalent bonds are critical in maintaining the three-dimensional structures of large molecules such as proteins and nucleic acids see Figure 2-1b.
Without hydrogen bonds DNA would have to exist as a different structure. Electron pairs shared between atoms of equal or very similar electronegativity constitute a nonpolar covalent bond eg HH or CH while electrons shared between atoms of unequal electronegativity constitute a polar covalent bond. Hydrogen bonding is a special type of chemical bond that involves dipole-dipole attraction between two or more dipolar molecules which are also referred to simply as dipoles.
The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds. One kind of nonpolar covalent bond that is very important. It carries both a positive and negative charge with the different charges separated by only a small space.
Types of chemical bonds including covalent ionic and hydrogen bonds and London dispersion forces. Ionic bonds are necessary because of their ability to form solution with water freeing up some of their ions to form other ionic compounds important to life. Hydrogen bonds are a great example of why I love the chemistry part of biochemistry so much.
Here are three reasons why hydrogen bonding is important. There are two types of covalent bonds. Peter C and Neil E.
High specific heat allows H2O to moderate temperature on Earth allowing life to live and reproduce. Covalent bonds are especially important since most carbon molecules interact primarily through covalent bonding. Noncovalent bonds also enable one large molecule to bind specifically but.
Ions and Ionic Bonds. In a true covalent bond the electronegativity values are the same eg H 2 O 3 although in practice the electronegativity values just need to be closeIf the electron is shared equally between the atoms forming a covalent bond then the bond is said to be nonpolar. The most important of these are the ionic covalent and metallic bonds.
Usually an electron is more attracted to one. Ionic bonds get dissocia. Living beings consist of complex molecules which support life functions and these bonds can be formed by covalent bonds only.
N and O can also be hydrogen bond donors and acceptors so they can create weak bonds important. They form the oxygen we breathe and help make up our living cells. Covalent bonds involve the sharing of electron pairs between atoms.
Hydrogen Bonds Are Dipole-Dipole Attraction. When electronegativity is below 15 strong covalent bonds can be formed. Ionic bonds result from a transfer of electrons while covalent bonds are formed by sharing.
Ionic bonds are stronger than covalent bonds hence it is harder to change covalent compounds. SYI1 EU SYI1B LO SYI1B1 EK Chemical bonds hold molecules together and create temporary connections that are essential to life. Covalent bonds are important to living things because they allow for the construction of stable complex biological molecules that can exist in an.
Covalent bonds are necessary for things like DNA cellular storage of glucose etc. Carbon is the most important element for living things as it can link with other carbon atoms and form long complex chains and molecules. From the tiny world of the nucleus and the principles of intercellular forces the properties of.
Home Blog Single Post. Covalent bonding allows molecules to share electrons with other molecules creating long chains of compounds and allowing more complexity in life. The biochemistry of living organisms requires.
Secondly what is the most important chemical bond. Nonpolar covalent bonds are extremely important in biology. In a polar covalent bond shown in Figure 1 the electrons are unequally shared by the atoms and are attracted more to one nucleus than the otherBecause of the unequal distribution of electrons between the atoms of different elements a slightly positive δ or slightly negative δ charge develops.
A High surface tension B The ability to dissolve polar substances C The ability of ice to float in water. The structure of DNA. Name one unique property of water explain why that property is so important to life.
Three types of chemical bonds are important in human physiology because they hold together substances that are used by the body for critical aspects of homeostasis signaling and energy production to name just a few important processes. The bond in which bonded atoms share electrons is called an _____. Google Classroom Facebook Twitter.
How is an ionic bond different than a covalent bond. In a covalent bond the atoms are bound by shared electrons. Both covalent and ionic bonds are important in the processes of life.
These are ionic bonds covalent bonds and hydrogen bonds.
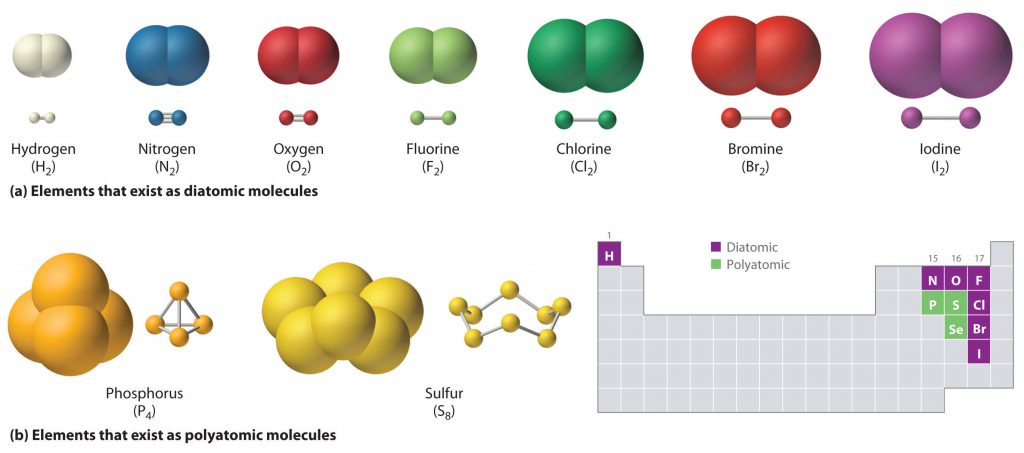
Ch103 Chapter 5 Covalent Bonds And Introduction To Organic Molecules Chemistry
Single And Multiple Covalent Bonds Article Khan Academy

10 Covalent Bond Examples In Real Life Studiousguy

10 Facts About Covalent Bonds The Habitat
Chemical Bonds Anatomy Physiology
10 Covalent Bond Examples In Real Life Studiousguy

Why Are Covalent Bonds Important In Living Things Study Com
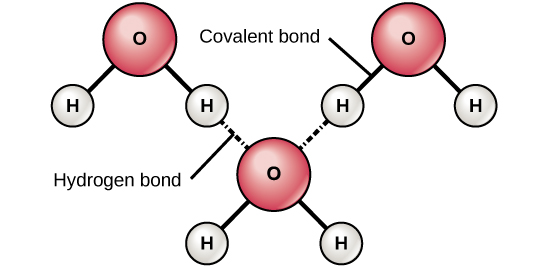
Why Life Depends On Water Biology For Non Majors I

Covalent Bonds Are The Sharing Of Electrons To Reach Octet And Ionic Are The Transfer Of Electrons From Chemistry Classroom Science Homework Teaching Chemistry

Chemical Bonds Anatomy And Physiology I

Chemical Bonds Anatomy And Physiology I
Chemical Bonds Anatomy Physiology
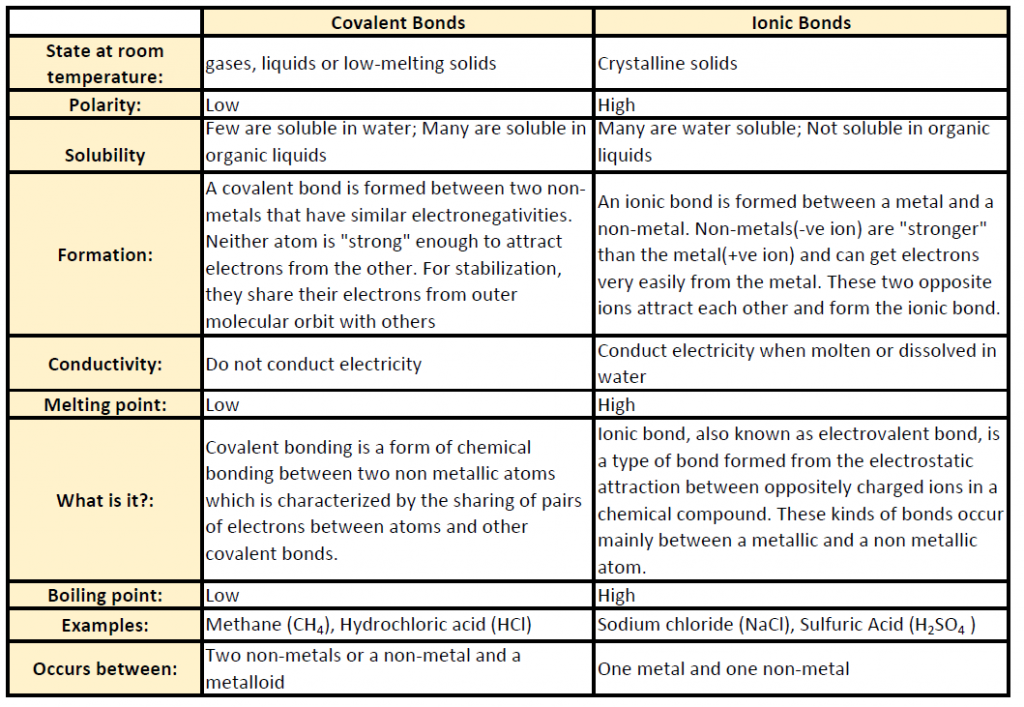
Ch103 Chapter 5 Covalent Bonds And Introduction To Organic Molecules Chemistry

10 Covalent Bond Examples In Real Life Studiousguy

Chapter 3 Water And Life Polar Covalent Bonds
Real Life Applications Chemical Bonding Atoms Electrons And Ions Electrons And Ions

Types Of Bonds And Orbitals Chemistry Education Chemistry Lessons Science Education

Ch150 Chapter 4 Covalent Bonds And Molecular Compounds Chemistry

Covalent Bonds Biology For Majors I

