OH MY.. Why Does Ice Have A Lower Density Than Water
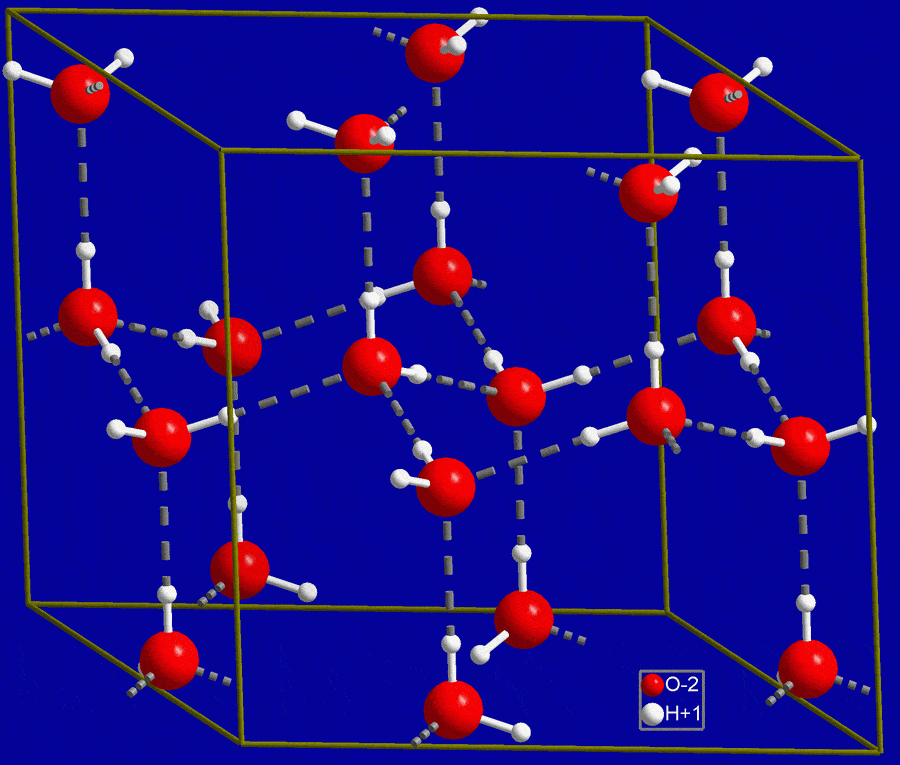
The water molecules low energy in their solid state prevents them from fulfilling the attraction of the hydrogen bonds and moving close to each other. In ice the hydrogen bonds hold the water molecules apart in an open lattice structure.
Meet Aeroice Scientists Just Engineered The Lowest Density Ice Crystals Ever
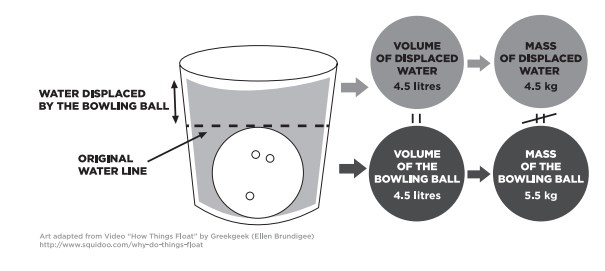
Because ice floats we can infer that ice must be less dense than water.
Why does ice have a lower density than water. One could argue that the reason that water density is a maximum at 4 ce0C 277 K is that the structure of liquid water is changing from that of ice. A substance floats if it is less dense or has less mass per unit volume than other components in a mixture. If water is frozen in a glass jar the glass jar breaks.
Water does exist in the form of ice or snow in the solid-state. It turns out that ice has a lower density than water and any object that has a lower density than the liquid form on which its kept in this case water will be able to float. What other observations or facts are known.
Each of the molecules has the same mass in each of the phases. But you must have observed that ice floats on water. From the image above notice how the ice molecules.
Thus the volume of ice is greater than water. Yes some ice is denser than water. When water freezes water molecules form a crystalline structure maintained by hydrogen bonding.
Water is considerably less dense than its solid counterpart ice and this unusual property is considered to be due to the hydrogen bonding in water. How bout this beastly answer. Solid water or ice is less dense than liquid water.
The molecules in water are affected by a phenomenon known as hydrogen bonding. For example if you toss a handful of rocks into a bucket of water the rocks which are dense compared to the water will sink. Answer 1 of 4.
Solid water or ice is less dense than liquid water. The mass per unit volume of a substance is called density density massvolume. Report 12 years ago.
A substance with low density than water. At zero degrees ie the temperature at which water turns into ice the density of water is actually quite low. The hydrogen bonding gives water a structure with considerable space between the molecules making it expand in size and become less dense in a solid state than in a liquid one.
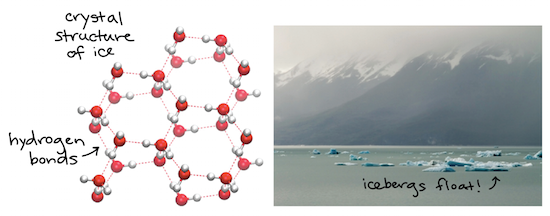
When water freezes its molecules lose energy and get stuck in a lattice structure in which they are farther apart from each other than in their liquid state thus making ice less dense than water. Heres an example of the crystalline structure of ice. In the first ice crystal there are spaces between some of the molecules which is not there in the second crystal structure.
A water molecule is a V-shaped molecule made up of one oxygen atom in the centre with a hydrogen atom on each side. These spaces are larger as compared to spaces in water molecules. Why does ice float.
5For other liquids solidification when the temperature drops includes the lowering of kinetic energy which allows molecules to pack more tightly and. The water which is less dense than the rocks will float. Water is the only substance on earth where its density is HIGHER when liquid and LOWER when solid.
Why Ice Floats. As this ice structure has a rather open lattice this is the reason its density is less at its freezing point than that of liquid water rather than being due to the high density of water. In the gasvapor phase molecules have very little interaction with each other and.
Why does ice have a lower density than water. Ice is less dense than liquid water which is a very unique property of water because usually the solid for most of a substance is more dense than the liquid form. Though ice is a solid it has large amount of space between its molecules.
From both of the above we infer that the volume of the ice. As the volume of a substance increases its density decreases. Hydrogen bonds link each water molecule to an.
Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart which lowers the density. The relative density of different phases of the same compound like water are purely determined by the number of molecules per volume. When water freezes to form ice some empty.
Why is ice less dense than water. If solids are denser than liquids why does ice float on water. Because water is denser than ice ice cubes float on the surface of water.
Because water is a special case. It has to do with how the H_2O molecules are hydrogen bonded to one another in the solid and liquid form. The density of ice is less than water.
A substance will float on top of another if its density is lower than the other substance. Water exists as water vapour or steam in the gaseous state and it exists in the form of water in its liquid state. Liquids generally have lower density as compared to solids but ice floats on water.
Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart which lowers the density. Density_ice 0934 gcm3 density_water 0997 gcm3 The difference between the densities has to do with how H_2O molecules are hydrogen bonded in solid ice versus liquid water form. Hence the density of ice is less than of water.
But ice has a lower density because when it forms its crystalline structure it actually introduces a significant amount of void space which wasnt present in liquid water. If a pop can freezes it will also burst. Liquids generally have lower density as compared to solids.
If you put pressure on regular ice and give it time to rearrange the molecules will move into a new crystal lattice which results in the ice being more dense than water. Why Does Ice Have a Lower Density Than Water.

Temperature And Density Chapter 3 Density Middle School Chemistry

Density Of Water Higher Than Density Of Ice

Density Of Water Higher Than Density Of Ice

Why Does Ice Form On The Top Of A Lake Science Questions With Surprising Answers

Floaters And Sinkers Science World
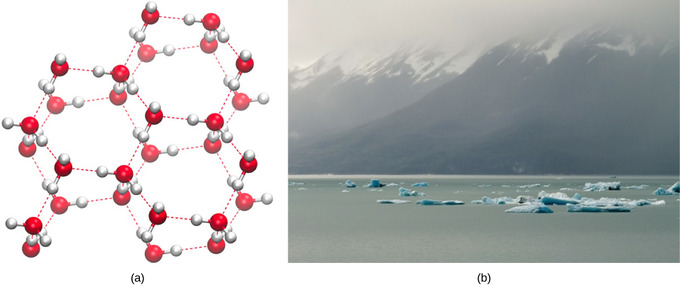
2 2b Water S States Gas Liquid And Solid Biology Libretexts

Density Poster Or Handout For 3rd 4th And 5th Graders Science Notebooks Learning Poster Upper Elementary Science
Density Temperature And Salinity Manoa Hawaii Edu Exploringourfluidearth

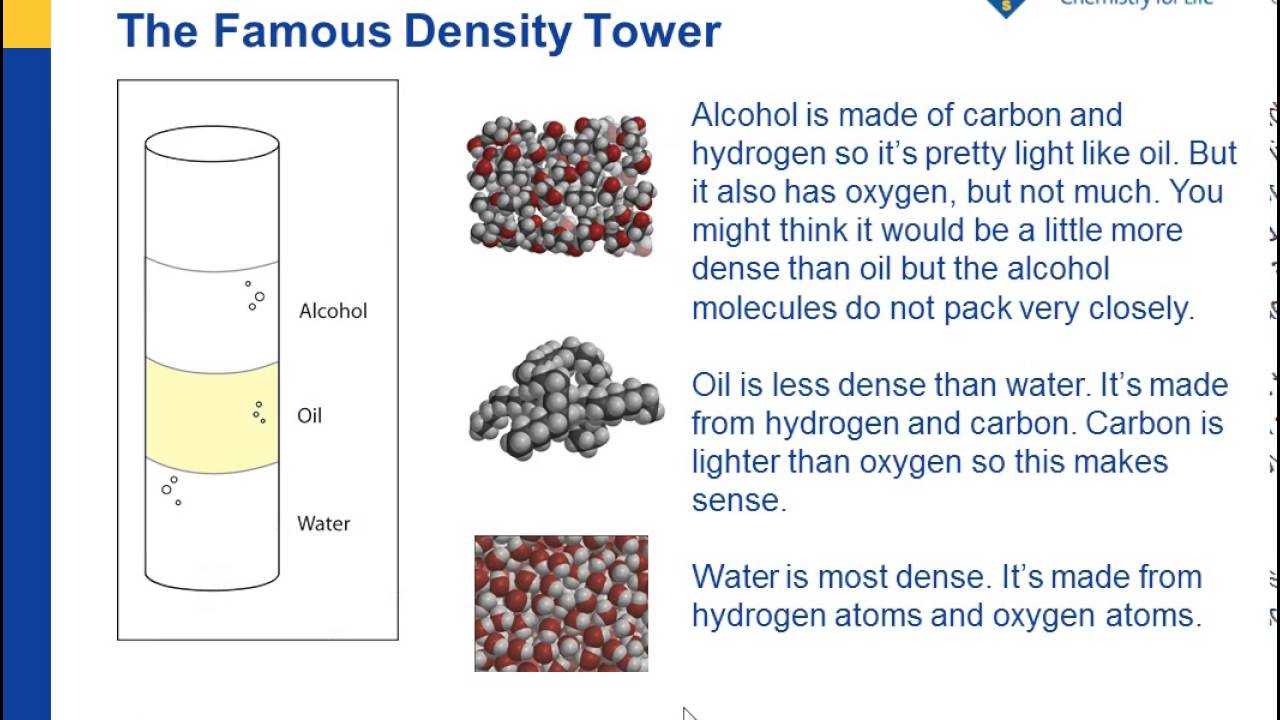
The Density Of Liquids American Chemical Society
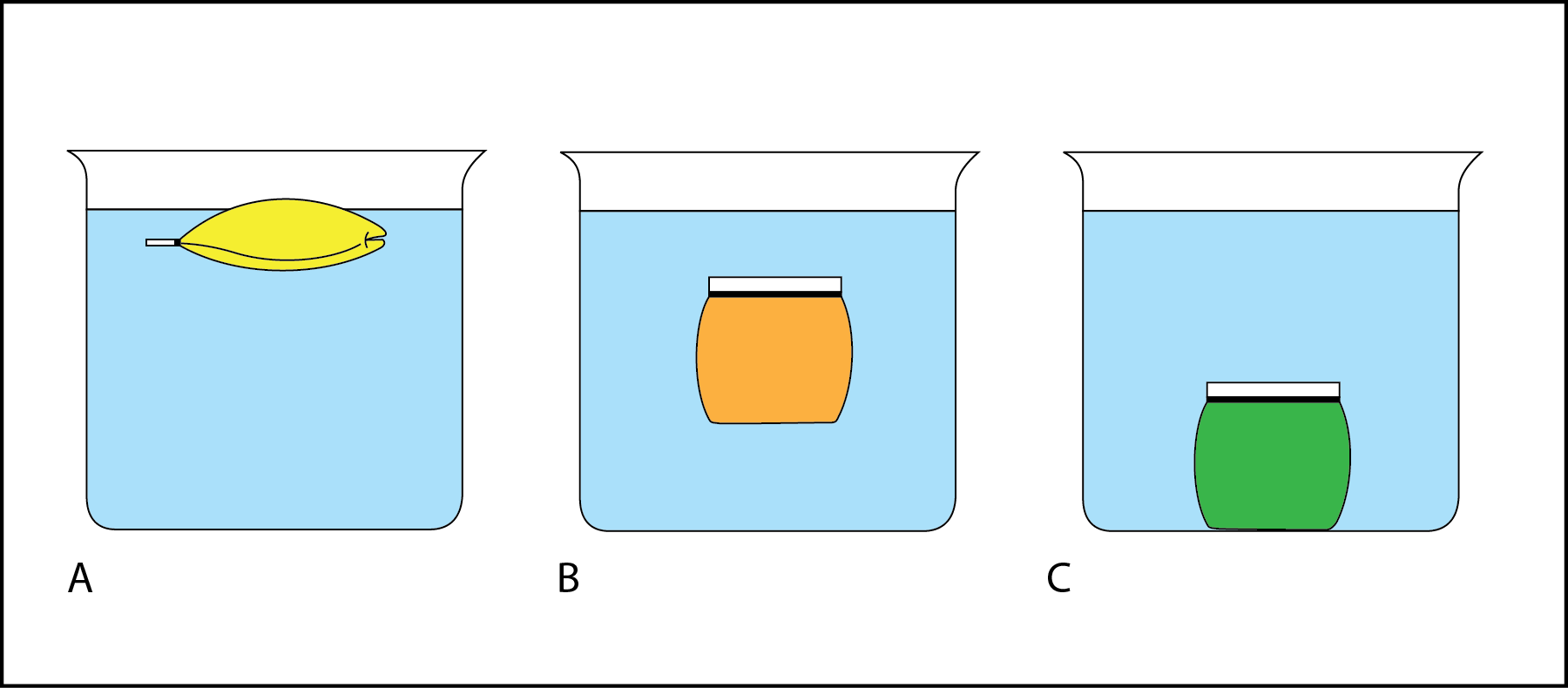
Floaters And Sinkers Science World
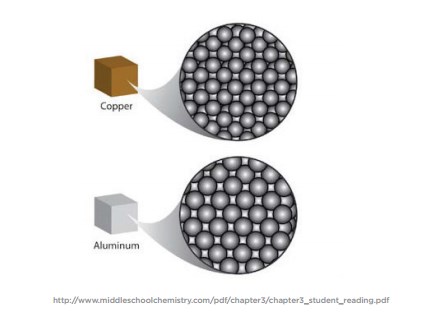
Density Sink And Float For Liquids Chapter 3 Density Middle School Chemistry
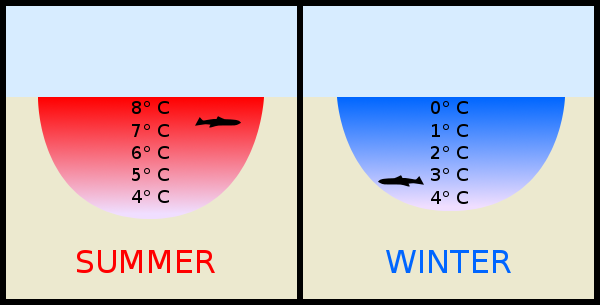
Why Does Ice Form On The Top Of A Lake Science Questions With Surprising Answers
Why Does Ice Have Less Density Than Water And Water Has A Maximum Density At 277 K Quora
Specific Heat Heat Of Vaporization And Density Of Water Article Khan Academy

Ice Is Born In Low Mobility Regions Of Supercooled Liquid Water Pnas